Shake to break… shake to make!
Utilizing 3D printing technology, Science within Reach (SWR) has designed an interactive model demonstrating self-assembly. SWR’s Self-Assembly Virus Kit is composed of virus subunits and magnets, representing the attractive forces between them. By shaking the model, the virus subunits bind and break apart in route to the most stable structure. Strength of the shaking is analogous to the temperature of the molecular environment. If it is too hot, the virus will break apart. If it is in the proper range, assembly will take place at an optimal pace.
Green Versus Red: A Lesson in Chirality
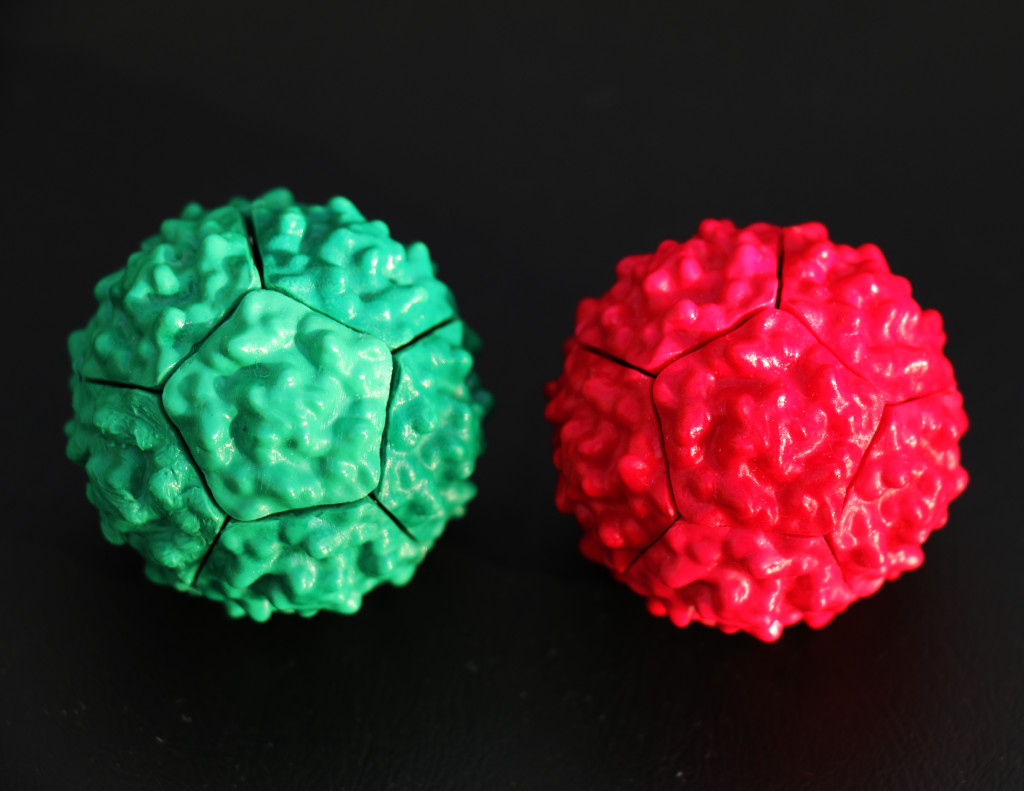
While our green and red viruses may look identical (disregarding color), they are actually chiral. This means they are mirror images of each other. Similarly, your hands are chiral. Though your hands look the same, there is no way you can twist or turn one to look exactly like the other. It’s why you can’t wear a right-handed glove on your left hand and vice versa.
In science, a pair of molecules that are chiral are called enantiomers. Since your hands are chiral, you could consider them enantiomers, too. Interestingly, because your hands are such good examples to explain chirality, scientists refer to one of the chiral molecules as “right-handed” and the other as “left-handed.”
Our green virus is right-handed and the red virus is left-handed. So, what do you think would happen if you put the red virus in with the green and started shaking? Would you get: A) two multicolored viruses, B) one giant red/green virus, or C) a separate green and a separate red virus? Check out our experiment below: multiple red and white chiral viruses mixed in vessel:
This video was made by Arthur Olson and Skylar Tibbits.
If you guessed C) a separate green and red virus, you are correct! Since they are enantiomers, they can’t wear each other’s gloves–or bind to each other because they are structurally different, though they look the same. Don’t believe us? See for yourself with a self-assembly virus kit.
